Earlier this month, Eat Well Global attended the 8th Annual Food Policy Impact Conference hosted by the American Society for Nutrition and the Institute of Food Technologists in Washington, D.C. The event, chaired by Dr. Taylor Wallace, covered a variety of topics from food labeling policy updates to the international food policy landscape. In addition to the engaging speakers and discussions, the conference hosted a discussion titled Controversy Panel on Cell-Cultured Meat. Speakers on this panel included Brian Ronholm, Senior Director of Regulatory Policy at Arent Fox LLP; Deepti Kulkarni, Partner at Sidley Austin LLP; and Jessica Almy, Director of Policy at the Good Food Institute.
Cell-cultured meat, also known as lab-grown meat or clean meat, is generated through a complex process using cells from healthy animals and combining it with other compounds. It is a process that scientists have performed in labs for years but has just now hit the consumer market. Emerging brands bringing these consumer products to market posit that creating and eating lab-grown meat is more sustainable, as it cuts down on greenhouse gas emissions and water consumption, and allows us to grow only the parts of the animal we would be consuming, thus reducing waste. Yet, there are many contentious issues that arise surrounding cell-cultured meat. The panelists discussed how both marketing and regulation controversies over lab-grown meat have transpired and continue to ensue debate.

Controversy over Regulation
The panelists first discussed the controversy of which federal agency had authority over regulating lab-grown meat. The FDA claims jurisdiction over ensuring the safety of the food supply, whereas the USDA plays a lead role in regulating meat, poultry, and egg products. The lab-grown meat advocates on the panel were adamant that the USDA not gain full authority over their product in fear that the department would suppress innovative technologies on behalf of the livestock industry. This so-called “turf war” over regulation of lab-grown meat between the FDA and the USDA that began in the summer of 2018 has finally resulted in a joint regulatory framework allowing both federal agencies to oversee different parts of the cell-cultured meat production process. The FDA will oversee the cell collection, cell banks, and cell growth and differentiation, while the USDA will regulate the production and food labeling of cell-cultured meats.

Controversy over Representation
At this point in the discussion, the panelists turned to their differing perspectives on consumer transparency and how these lab-grown meats should be labeled and marketed to consumers. Jessica Almy, Director of Policy at the Good Food Institute, stated that her organization believed that lab-grown meat should be labeled simply as ‘meat’ rather than phrases like ‘clean meat’ or ‘lab-grown meat’. While the nutritious value of lab-grown meat compared to real meat has not been fully evaluated, the discussion around labeling remains a contentious topic. As of February 2019, a handful of states are attempting to pass laws requiring the clear labeling of lab-grown meat, with several plant-based organizations pushing back, worried about the misrepresentation of their products. So, what is next for lab-grown meat, and what can we expect from the USDA regarding labeling? We’ll be keeping our finger on the pulse of this ever-evolving regulatory landscape.
Related News
June 30, 2025
Health-Conscious Consumer Segmentation Report
Get actionable insights to meaningfully engage today’s diverse health-conscious consumers — and better align your brand’s communication and…
January 25, 2021
Eat Well Connect Voices: Tessa Nguyen
In this latest installment of our Eat Well Connect Voices Q&A, Tessa Nguyen, RD, LDN, of Taste Nutrition Consulting shared her thoughts on what…
November 27, 2020
The Conference Discussing the Future of Food, Drink and Nutrition Goes Virtual
This October, Food Matters Live held its first virtual conference. The conference focused on identifying opportunities in a changing consumer-driven…
November 19, 2020
Partner Highlights: Global Alliance for Improved Nutrition
In the global fight against hunger and malnutrition, few organizations are as impactful as the Global Alliance for Improved Nutrition (GAIN). Since…
November 18, 2020
Eat Well Connect Voices: EatWell Exchange
In this latest Eat Well Connect Voices Q&A, Ashley Carter, RD, LDN and Jasmine Westbrooks, MS, RD, LDN of EatWell Exchange Inc. share their…
October 12, 2020
Next Normal: Optimal Nutrition for a Pandemic Reality
The COVID-19 pandemic has had a profound impact on us all, and the food, nutrition and healthcare industries are no exception. While nutrition…
October 1, 2020
Maintaining Sustainability Commitments While Protecting Profits
Earlier this month, I had the opportunity to present at the 2020 Vitafoods Europe Virtual Expo and discuss how consumer packaged goods companies can…
September 20, 2020
Eat Well Connect Voices: Shahzadi Devje, RD
We're thrilled to connect with Eat Well Connect (EWC) member Shahzadi Devje, RD as she shares her thoughts on using nutrition communication as a tool…
June 29, 2020
Nutrition Communication in the Time of COVID
According to a recent survey conducted by the International Food Information Council (IFIC), consumers consider health professionals and registered…
June 4, 2020
Impactful Communication: Meaningful Messages to Inspire Change
Do you want to be more impactful in your work as a dietitian or nutrition professional? Learning to effectively build a message and communicate it to…
May 19, 2020
Putting Nutrition to Work: Acting on Our Vision that Good Nutrition is Good Business
Workforce nutrition programs offer benefits for employees as well as businesses, such as reduced absenteeism, increased productivity and improved…
September 18, 2019
Exploring the Ongoing Evolution of Food, Sustainability and Nutrition at The Future of Food Summit
As Eat Well Global further establishes its unique position empowering global change agents in our field, we actively seek more perspectives and…
August 7, 2019
Climate, Culture & Economics: Expanding our Nutrition Perspective at the Asian Congress of Nutrition
Hosted by the Federation of Asian Nutrition Societies (FANS) every four years, the Asian Congress of Nutrition is Asia’s prime convening of food and…
June 23, 2019
Marrying Medicine and Nutrition: Health Meets Food Conference
Hosted by the Tulane University School of Medicine, Health Meets Food’s 6th annual Culinary Medicine conference brought together primarily physicians…
April 25, 2019
The Power of Pairing, Partnerships and Produce at PBH Consumer Connection Summit
Launching a fresh new campaign, “Have a Plant”, PBH connected consumers’ increasing interest with adding more plants to their diet to their ongoing…
April 15, 2019
Accelerating Healthier Futures Together
Over the years, we’ve seen Partnership for a Healthier America (PHA) embark on new initiatives, bringing along their star-studded friends to amplify…
April 8, 2019
The 2nd Annual Culinary Nutrition Conference
Chefs and nutrition professionals came together for the 2nd Annual Culinary Nutrition Conference this month at the Institute of Culinary Education…
April 3, 2019
Internet of Food Brought to Life at IC-Foods 2019
When the concept of the “Internet of Food” comes up, people often ask, “don’t we already have an internet? And can’t you find food there?” The…
March 22, 2019
The Chicago Council on Global Affairs: Global Food Security Symposium 2019
For the past 10 years, The Chicago Council on Global Affairs has been convening key stakeholders from the public, private and NGO sectors at their…
December 20, 2018
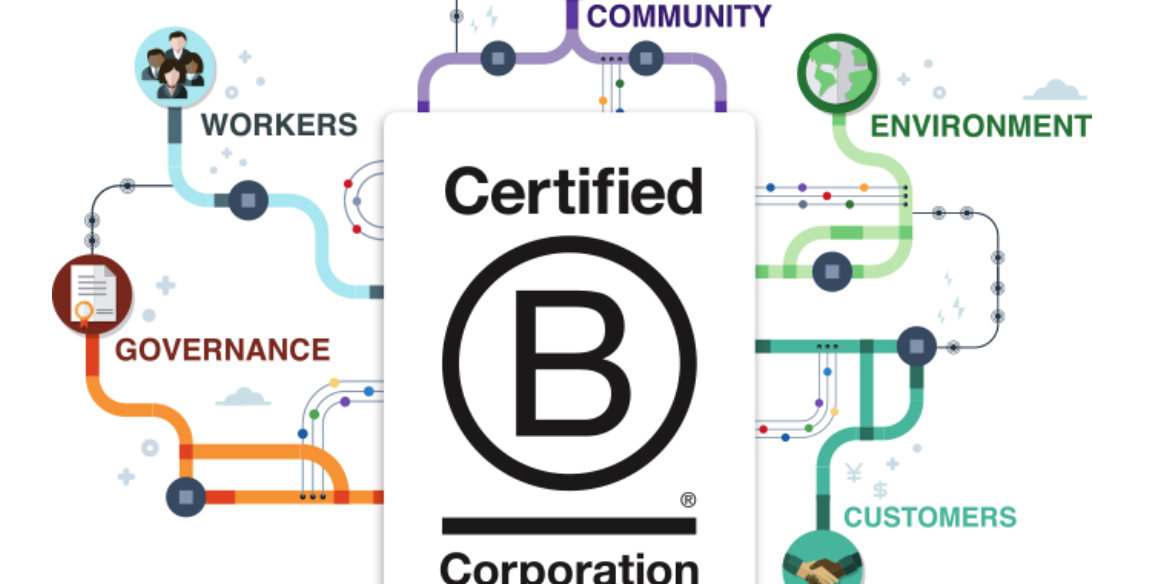
Eat Well Global Proudly Announces B Corp Certification
We are excited to announce Eat Well Global is now officially B CorpTM certified!
December 12, 2018
PRESS RELEASE: Eat Well Global Joins Corporate Leaders in Social Responsibility with Recent B Corporation® Certification
Certified B Corporations® include businesses that voluntarily meet the highest standards of social and environmental performance, public…
November 7, 2018
PRESS RELEASE: Healthy Marketing Team and Eat Well Global join forces to help industry address rapidly changing consumer nutrition market in North America
The international brand strategy and nutrition innovation agency, Healthy Marketing Team (HMT), announce their cooperation with Eat Well Global, Inc…
October 25, 2018
Eat Well Global Brings Nutrition Communication to the Forefront at EFAD 2018
Eat Well Global participated in the 40th Anniversary European Federation of the Associations of Dietitians conference in Rotterdam, the Netherlands.…
September 6, 2018
“Nutrition is Everyone’s Business”: Insights from the SDG Conference – Partnerships are Critical to Ending Hunger
This August, Eat Well Global attended the SDG-Conference ‘Towards Zero Hunger: Partnerships for Impact’ at Wageningen University & Research in the…
August 20, 2018
Communicating to Influence at EFAD Conference 2018
For dietitians, the ability to communicate effectively is an essential skill. Whether working in a hospital, school, food company or even your own…
May 11, 2018
Partnership for a Healthier America Summit Brings 360 Degree View of Better Health
The 2018 PHA Summit brought together diverse stakeholders to support their mission: make the healthy choice, the easy choice.
March 14, 2018
Food Tank 2018 Highlights Need for Farmer Support
At the recent Food Tank Summit in Washington, D.C., farmers, senators, members of Congress, nonprofit and business leaders, media, and young…
November 22, 2017
Dubai International Food Safety Conference Brings Big Data to the Plate
Technology and food safety were the name of the game at the 11th Annual Dubai International Food Safety Conference, which took place November 19-21,…
June 15, 2017
Harvesting Insights from the Next Generation of Food Security Innovators: Thought For Food Global Summit 2017
Have you ever heard of choco-panda ice cream? Me neither. How about a superhero on a mission to teach kids about nutrition and food security? If…
May 9, 2017
Eat Well Global on Influencer Trust at Food Vision Asia
It is no news: achieving a healthy lifestyle is top of mind for millions of people around the globe and social media plays a big role in driving…
March 17, 2017
Eat Well Global Talks Authenticity in Food Navigator Interview
With an apparent rise in distrust of science and facts coupled with an increase in social media marketing, there has been a shift in consumer…
March 14, 2017
Eat Well Global at Food Vision in London!
Trend makers, marketing and communication specialists and industry leaders gathered at the Food Vision event in London, from 1-3 March. Among the…
January 26, 2017
Eat Well Global addresses health influencers & brand power in Food Navigator article
In times where consumer demand for transparency and authenticity intersect with fast pace influencer marketing, reaching consumers through trusted…
January 17, 2017
Eat Well Global talks 2017 global food trends on Food Navigator-USA’s Podcast
To kick off 2017, Julie Meyer, co-founder of Eat Well Global and GA4HNC, participated in two episodes of Food Navigator-USA's Soup-to-Nuts-Podcast.
January 2, 2017
Convenient, Committed and Cool: 2016 NY Produce Show Review
Today's consumer seeks more from their produce and industry is delivering
December 20, 2016
The American Public’s Opinion on Food Science
A recent survey by the Pew Research Center asked Americans about their opinions regarding the science behind food and health recommendations and…
December 12, 2016
What Americans Think About Healthy Eating
With impending changes in the political climate, many people have been asking what this means for the future of food policy.
November 22, 2016
Food Vision USA: Innovation, Disruption and What’s Next
Leaders from start-ups to trend makers to innovative disruptors share their thoughts on the big question: what's next?
November 14, 2016
Want people to eat more fruits and vegetables? Inspire them.
As a nutritionist, I love this statement. As a communicator, I couldn’t agree more.
October 5, 2016
ICD 2016: How Dietitians Can Impact Sustainable Eating
What role can (and should) dietitians play in the sustainability dialogue? We went to Granada to find out!
August 17, 2016
The Future Is Now: New Harvest 2016
Several hundred (309 to be specific) researchers, entrepreneurs, visionaries, futurists and interested parties gathered under sunny skies in San…